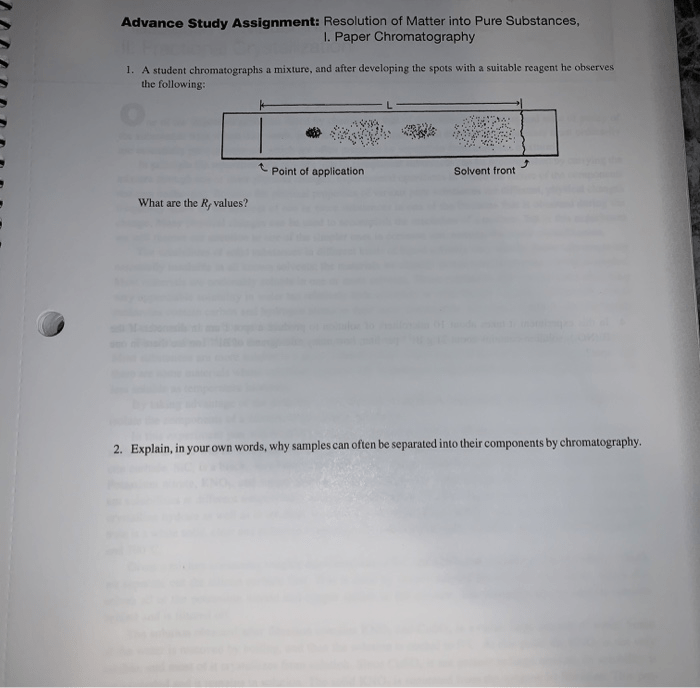
Resolution of matter into pure substances fractional crystallization answer key – Fractional crystallization is a technique used to separate and purify substances based on their different solubilities. This answer key provides a comprehensive overview of the process, its applications, and its significance in various fields, particularly in the pharmaceutical industry.
This guide delves into the concept of resolution of matter, exploring the factors that affect fractional crystallization’s success and comparing it to other separation methods. It also examines the types of pure substances and their relevance to fractional crystallization.
Resolution of Matter into Pure Substances: Fractional Crystallization
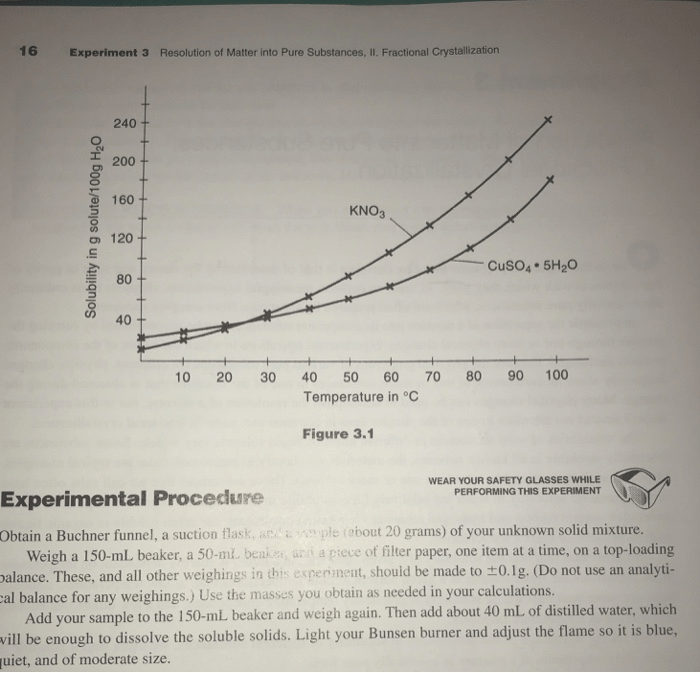
Fractional crystallization is a technique used to separate a mixture of substances by crystallizing and then separating the crystals based on their different solubilities.
Fractional Crystallization
The process of fractional crystallization involves the following steps:
- The mixture is dissolved in a solvent.
- The solution is cooled slowly, causing the least soluble component to crystallize out.
- The crystals are separated from the solution by filtration.
- The crystals are then redissolved and the process is repeated until the desired purity is achieved.
The success of fractional crystallization depends on several factors, including the difference in solubility between the components, the rate of cooling, and the size of the crystals.
An example of a fractional crystallization experiment is the separation of a mixture of sodium chloride and potassium chloride. Sodium chloride is less soluble than potassium chloride, so it will crystallize out first. The crystals can then be separated from the solution by filtration and the process can be repeated until the desired purity is achieved.
Pure Substances
A pure substance is a substance that has a constant composition and distinct properties. Pure substances can be either elements or compounds.
There are two main types of pure substances: homogeneous and heterogeneous.
- Homogeneous pure substances have the same composition throughout.
- Heterogeneous pure substances have different compositions in different parts.
Fractional crystallization can be used to obtain pure substances by separating the different components of a mixture.
Resolution of Matter, Resolution of matter into pure substances fractional crystallization answer key
The resolution of matter is the process of separating a mixture into its individual components.
There are several different methods of resolving matter, including:
- Filtration
- Distillation
- Chromatography
- Fractional crystallization
Fractional crystallization is a particularly useful method for separating mixtures of solids.
Applications of Fractional Crystallization
Fractional crystallization has a wide range of applications in industry, including:
- The purification of chemicals
- The production of pharmaceuticals
- The separation of minerals
Fractional crystallization is a versatile and powerful technique that can be used to separate a wide variety of mixtures.
FAQ: Resolution Of Matter Into Pure Substances Fractional Crystallization Answer Key
What is the principle behind fractional crystallization?
Fractional crystallization relies on the varying solubilities of different substances in a solvent at different temperatures.
How does fractional crystallization contribute to obtaining pure substances?
By selectively crystallizing and separating substances based on their solubilities, fractional crystallization allows for the isolation of pure components from a mixture.
What are the factors that influence the efficiency of fractional crystallization?
Factors such as temperature, solvent selection, and the initial composition of the mixture can impact the success of fractional crystallization.