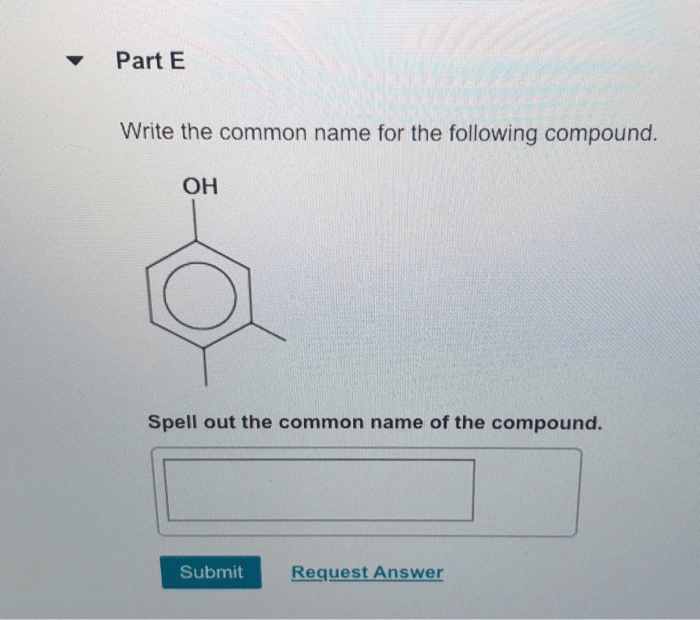
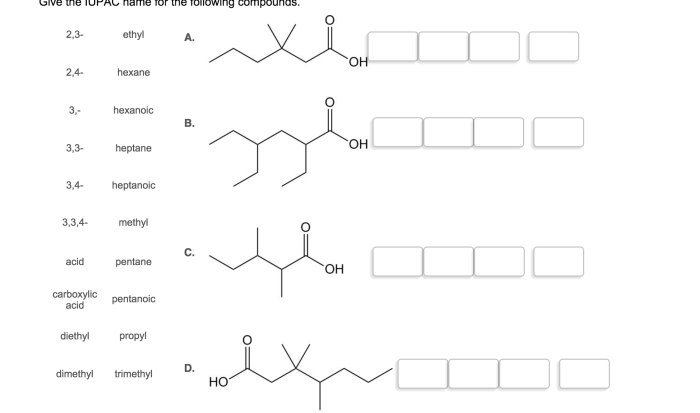
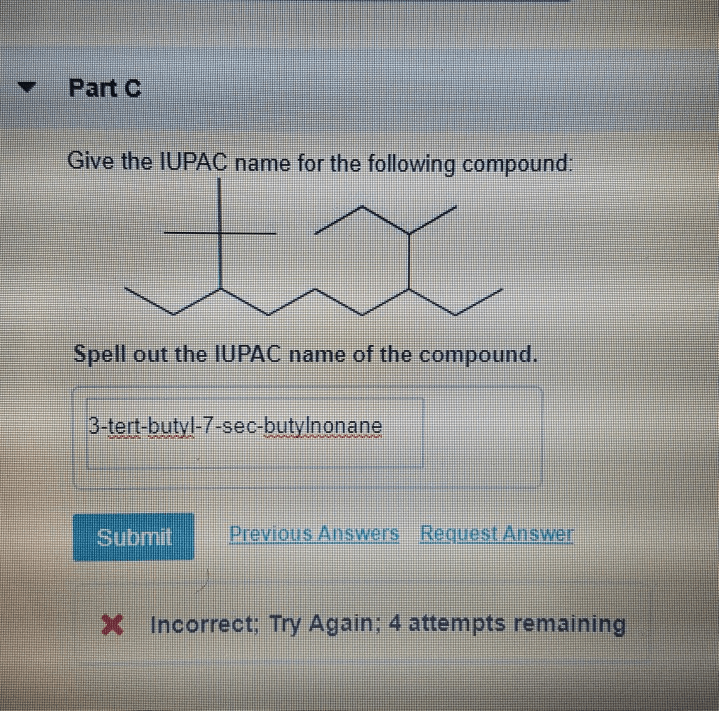
Spell out the iupac name of the compound. – Delve into the fascinating world of IUPAC nomenclature and master the art of spelling out the IUPAC name of any organic compound. This comprehensive guide will equip you with the knowledge and tools to navigate the intricacies of IUPAC rules and confidently name even the most complex molecules.
IUPAC Nomenclature Rules
IUPAC nomenclature is a systematic method for naming chemical compounds. It is based on a set of rules developed by the International Union of Pure and Applied Chemistry (IUPAC). These rules provide a consistent and unambiguous way to identify and name compounds.
General Principles of IUPAC Nomenclature
- The name of a compound is based on its structure.
- The name consists of a root word that indicates the parent chain and a suffix that indicates the functional group.
- Prefixes are used to indicate the number and position of substituents.
Specific Rules for Naming Organic Compounds
- The parent chain is the longest continuous chain of carbon atoms in the compound.
- The root word for the parent chain is based on the number of carbon atoms in the chain.
- The suffix for the functional group is based on the type of functional group.
- Prefixes are used to indicate the number and position of substituents.
Spelling Out IUPAC Names
To spell out the IUPAC name of a compound, follow these steps:
- Identify the parent chain.
- Identify the functional group.
- Add the root word for the parent chain and the suffix for the functional group.
- Add prefixes to indicate the number and position of substituents.
For example, the IUPAC name of the compound CH3CH2CH2OH is ethanol.
Functional Groups
Functional groups are groups of atoms that give organic compounds their characteristic properties.
- Prefix: The prefix indicates the number of carbon atoms in the functional group.
- Suffix: The suffix indicates the type of functional group.
| Functional Group | Prefix | Suffix |
|---|---|---|
| Alkyl | Alk- | -ane |
| Alkenyl | Alken- | -ene |
| Alkynyl | Alcyn- | -yne |
| Alcohol | – | -ol |
| Aldehyde | – | -al |
| Ketone | – | -one |
| Carboxylic acid | – | -oic acid |
Alkyl Groups
Alkyl groups are groups of atoms that are derived from alkanes.
The IUPAC name of an alkyl group is based on the number of carbon atoms in the group.
| Number of Carbon Atoms | IUPAC Name |
|---|---|
| 1 | Methyl |
| 2 | Ethyl |
| 3 | Propyl |
| 4 | Butyl |
| 5 | Pentyl |
Alkenes and Alkynes, Spell out the iupac name of the compound.
Alkenes are hydrocarbons that contain a carbon-carbon double bond.
Alkynes are hydrocarbons that contain a carbon-carbon triple bond.
The IUPAC name of an alkene or alkyne is based on the number of carbon atoms in the parent chain and the location of the double or triple bond.
For example, the IUPAC name of the compound CH3CH=CH2 is propene.
Aromatic Compounds
Aromatic compounds are cyclic compounds that contain a benzene ring.
The IUPAC name of an aromatic compound is based on the number of carbon atoms in the ring and the location of any substituents.
For example, the IUPAC name of the compound C6H5CH3 is toluene.
Branched Compounds
Branched compounds are compounds that have one or more side chains.
The IUPAC name of a branched compound is based on the name of the parent chain and the names of the substituents.
For example, the IUPAC name of the compound CH3CH(CH3)CH2CH3 is 2-methylbutane.
Polyfunctional Compounds
Polyfunctional compounds are compounds that contain two or more functional groups.
The IUPAC name of a polyfunctional compound is based on the names of the functional groups and the location of the functional groups on the parent chain.
For example, the IUPAC name of the compound CH3CH2CH(OH)COOH is 3-hydroxybutanoic acid.
Stereochemistry
Stereochemistry is the study of the three-dimensional structure of molecules.
The IUPAC nomenclature for stereochemistry uses a set of prefixes to indicate the relative configuration of atoms or groups of atoms in a molecule.
For example, the prefix Rindicates that a group is oriented to the right of a reference plane, while the prefix Sindicates that a group is oriented to the left of a reference plane.
Common Names vs. IUPAC Names
Common names are names that are commonly used for certain compounds.
IUPAC names are systematic names that are based on the rules of IUPAC nomenclature.
Common names are often easier to remember than IUPAC names, but they can be ambiguous.
IUPAC names are unambiguous and they can be used to identify any compound.
Common Queries: Spell Out The Iupac Name Of The Compound.
What is the purpose of IUPAC nomenclature?
IUPAC nomenclature provides a systematic and unambiguous way to name chemical compounds, ensuring consistency and clarity in scientific communication.
How do I determine the IUPAC name of an alkyl group?
Alkyl groups are named by replacing the -ane suffix of the parent alkane with -yl. The number of carbon atoms in the alkyl group determines the prefix.
What are the rules for naming branched compounds?
Branched compounds are named by identifying the longest carbon chain as the parent chain and naming the branches as substituents. The substituents are listed in alphabetical order, followed by the parent chain name.